-
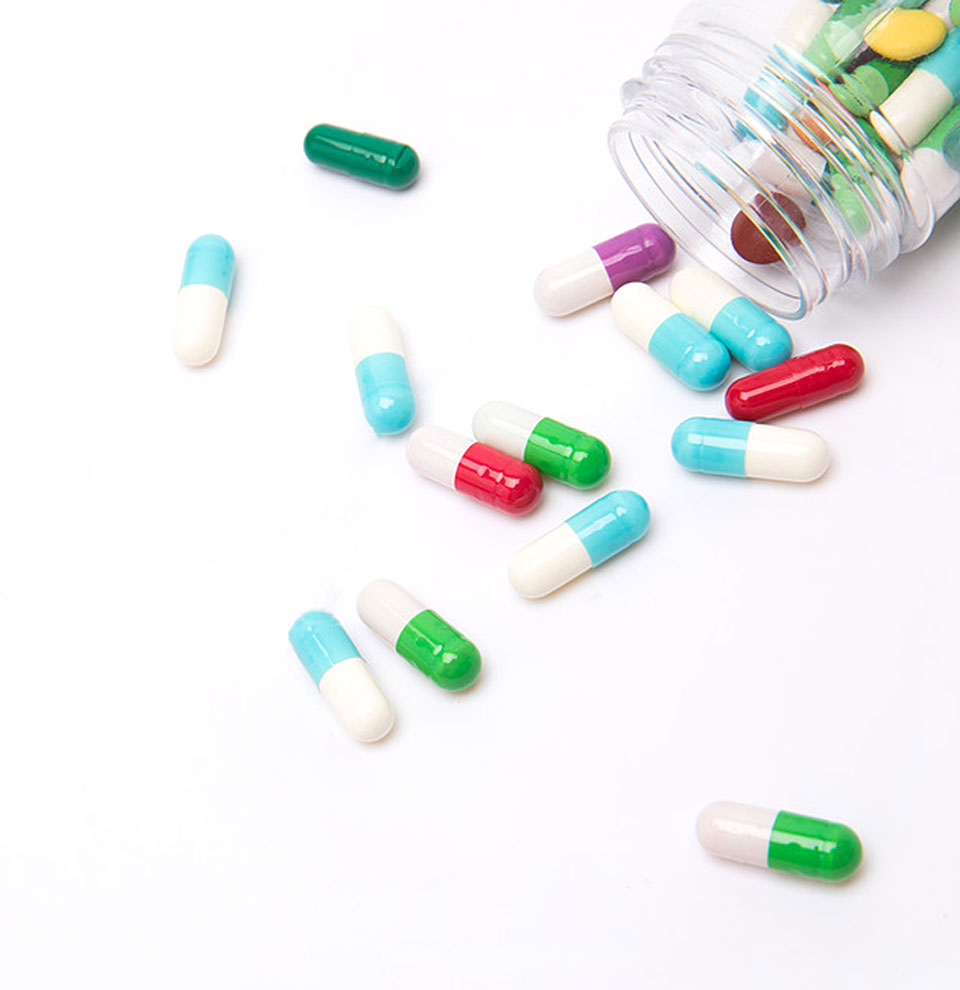
Can HPMC empty capsules use moisture-proof capsule materials?HPMC empty capsules can use moisture-proof capsule materials when it is necessary to improve capsule stability and extend product shelf life. HPMC itself is sensitive to moisture, and prolonged exposu...Read More
-
Will HPMC empty capsules deform or disintegrate in high temperature and high humidity environments?1. Moisture absorption and physical deformation of HPMC capsules hygroscopic mechanismHPMC molecular chains contain hydrophilic hydroxyl groups, which absorb moisture when humidity exceeds 60%, result...Read More
-
Can HPMC empty capsules provide precise dosage?HPMC empty capsules can indeed provide precise dosage. HPMC empty capsules, as a commonly used pharmaceutical excipient, have a wide range of applications in the field of food and medicine. Its unique...Read More
-
Do halal capsules need to completely ban pork and pork products?Yes, halal capsules must be completely prohibited from containing pork and pork products. Pork and pork products are considered unclean (halam), and according to Islamic law, Muslims are not allowed t...Read More
-
Can HPMC empty capsules be left unused for a long time?HPMC empty capsules can be left unused for a long time, but they need to be stored under appropriate conditions. Specifically, the following factors need to be noted:1. Storage conditions: HPMC empty ...Read More
-
Can I use HPMC empty capsules to show the ingredients of a formula?Yes, HPMC empty capsules are very suitable for displaying formula ingredients, for the following reasons:1. Transparent shell: HPMC empty capsules are usually transparent, and the contents inside the ...Read More
-
Can HPMC empty capsules use moisture-proof capsule materials?HPMC empty capsules can use moisture-proof capsule materials when it is necessary to improve capsule stability and extend product shelf life. HPMC itself is sensitive to moisture, and prolonged exposu...Read More
-
Will HPMC empty capsules deform or disintegrate in high temperature and high humidity environments?1. Moisture absorption and physical deformation of HPMC capsules hygroscopic mechanismHPMC molecular chains contain hydrophilic hydroxyl groups, which absorb moisture when humidity exceeds 60%, result...Read More
-
Can HPMC empty capsules provide precise dosage?HPMC empty capsules can indeed provide precise dosage. HPMC empty capsules, as a commonly used pharmaceutical excipient, have a wide range of applications in the field of food and medicine. Its unique...Read More
-
Do halal capsules need to completely ban pork and pork products?Yes, halal capsules must be completely prohibited from containing pork and pork products. Pork and pork products are considered unclean (halam), and according to Islamic law, Muslims are not allowed t...Read More
-
Can HPMC empty capsules be left unused for a long time?HPMC empty capsules can be left unused for a long time, but they need to be stored under appropriate conditions. Specifically, the following factors need to be noted:1. Storage conditions: HPMC empty ...Read More
-
Can I use HPMC empty capsules to show the ingredients of a formula?Yes, HPMC empty capsules are very suitable for displaying formula ingredients, for the following reasons:1. Transparent shell: HPMC empty capsules are usually transparent, and the contents inside the ...Read More
-
Can HPMC empty capsules use moisture-proof capsule materials?HPMC empty capsules can use moisture-proof capsule materials when it is necessary to improve capsule stability and extend product shelf life. HPMC itself is sensitive to moisture, and prolonged exposu...Read More
-
Will HPMC empty capsules deform or disintegrate in high temperature and high humidity environments?1. Moisture absorption and physical deformation of HPMC capsules hygroscopic mechanismHPMC molecular chains contain hydrophilic hydroxyl groups, which absorb moisture when humidity exceeds 60%, result...Read More
-
Can HPMC empty capsules provide precise dosage?HPMC empty capsules can indeed provide precise dosage. HPMC empty capsules, as a commonly used pharmaceutical excipient, have a wide range of applications in the field of food and medicine. Its unique...Read More
- Home
-
About Us
Why Choose Us?We firmly pursue for high technology, high quality, strict management and high efficiency! We solemnly promise to provide products and high-quality service. We look forward to cooperating with you.
 Coming From China, Marketing To The World.
Coming From China, Marketing To The World. - Products
- Contact Us



 English
English 中文简体
中文简体 русский
русский




























