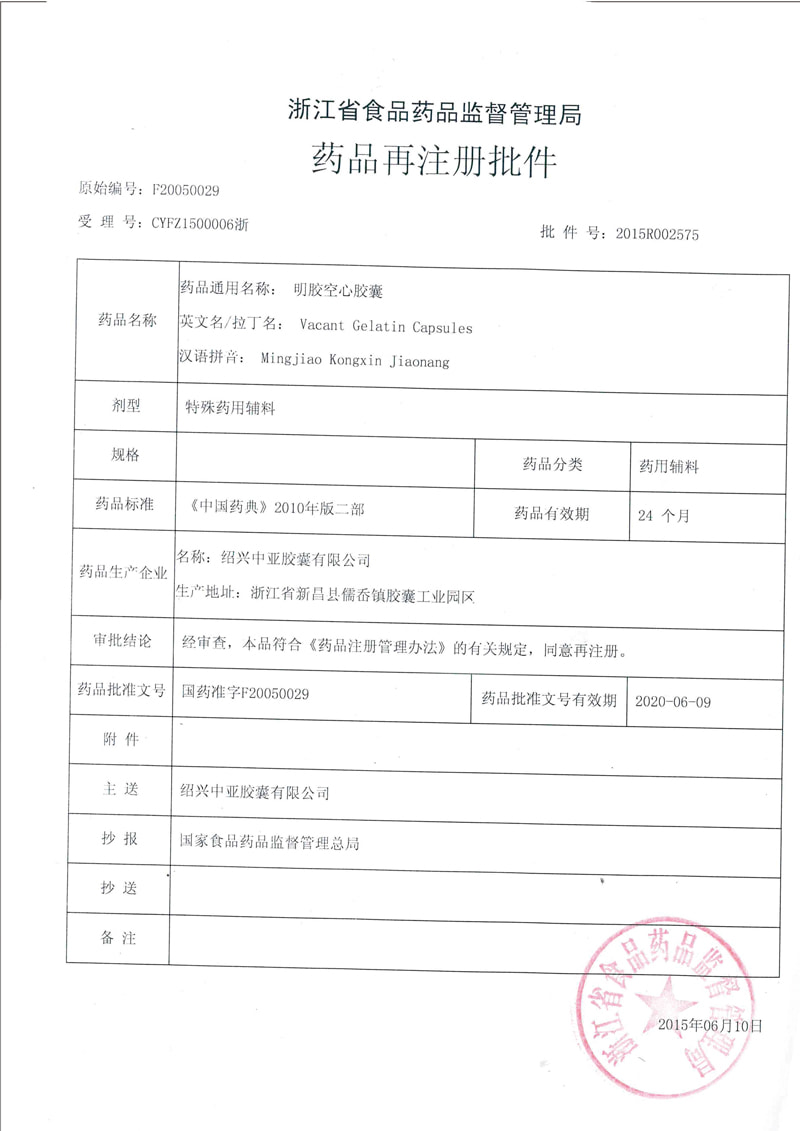
-
Gelatin Vacant Capsules are prepared from gelatin with certain amounts of pigments, Titanium dioxide, which general are transparent, semitransparent, or opaque.
Gelatin Vacant Capsules have advantages of efficiently masking taste, wide range of biologic applications, beautiful looking and easy to swallow, which are often served as pharmaceutical excipients and disintegrated and absorbed mainly at stomach.
Gelatin Vacant Capsules are modelled as 00#, 0#, 0# plus, 1#, 1# plus, 2#, 3#, 4# and more that meeting requirements of all kinds of pharmaceutical packaging.
We provide capsule products with circumferential printing, axial printing, dual-color printing and more. We also offer special customizations for clients.
About Company
Click The Color You Want, See What Happens.
CAP COLOR
BODY COLOR
News Center
Industry Knowledge Extension
Contact Us